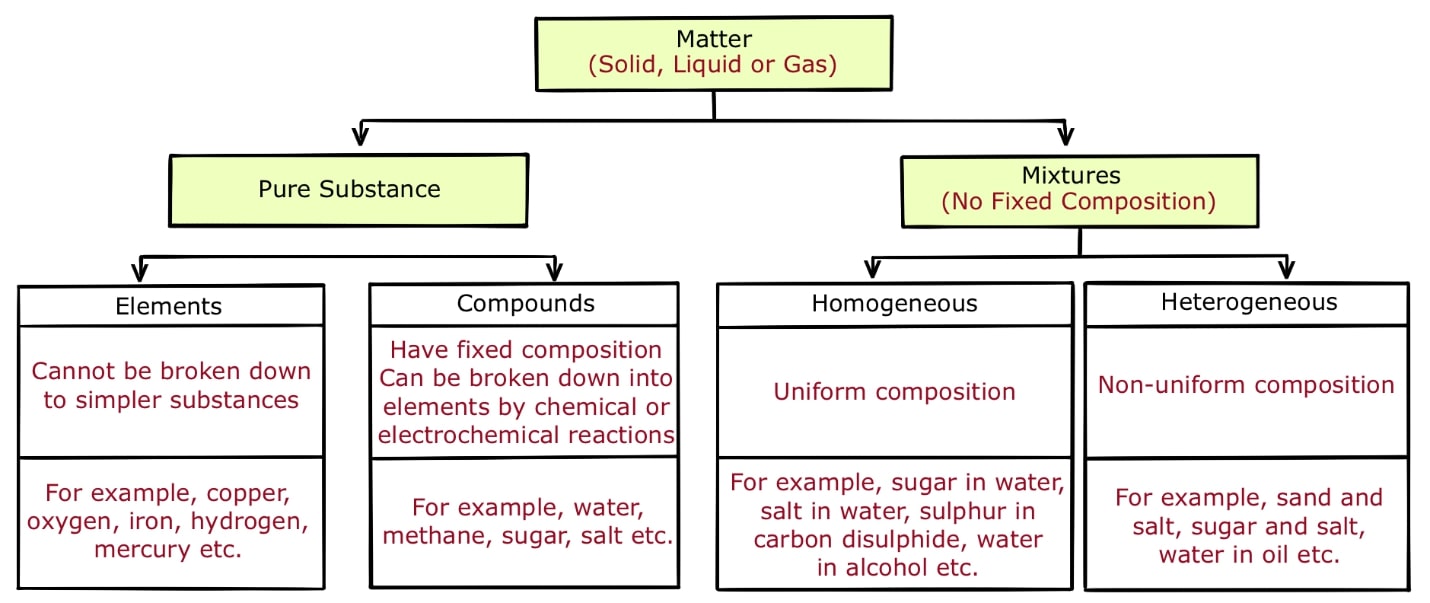
Mixture Examples Class 9 . A mixture can be separated into its constituents by physical processes. anne marie helmenstine, ph.d. the three phases or states of matter are gas, liquid, and solid. by combining two or more substances, a mixture is produced. The various substances present in a mixture are known as “constituents of the mixture” or “components of a mixture”. In chemistry, a mixture is a. A homogeneous solution tends to be identical, no matter how you sample. A mixture is a substance. We are able to see oil and water clearly separately in the. Updated on may 01, 2024. Mixture of oil and water is a heterogeneous mixture. When you combine two or more materials, you form a mixture. a mixture is a material that is made up of two more chemical compounds or substances that do not combine together.
from unacademy.com
In chemistry, a mixture is a. anne marie helmenstine, ph.d. the three phases or states of matter are gas, liquid, and solid. Updated on may 01, 2024. When you combine two or more materials, you form a mixture. by combining two or more substances, a mixture is produced. Mixture of oil and water is a heterogeneous mixture. a mixture is a material that is made up of two more chemical compounds or substances that do not combine together. The various substances present in a mixture are known as “constituents of the mixture” or “components of a mixture”. A mixture can be separated into its constituents by physical processes.
Science Class 9 Pure Substance vs Mixture UPSC Note on Science Class
Mixture Examples Class 9 In chemistry, a mixture is a. We are able to see oil and water clearly separately in the. In chemistry, a mixture is a. When you combine two or more materials, you form a mixture. anne marie helmenstine, ph.d. Updated on may 01, 2024. a mixture is a material that is made up of two more chemical compounds or substances that do not combine together. A homogeneous solution tends to be identical, no matter how you sample. A mixture can be separated into its constituents by physical processes. The various substances present in a mixture are known as “constituents of the mixture” or “components of a mixture”. Mixture of oil and water is a heterogeneous mixture. A mixture is a substance. the three phases or states of matter are gas, liquid, and solid. by combining two or more substances, a mixture is produced.
From www.youtube.com
Compound and Mixture Class 9 What is the Difference between Compound Mixture Examples Class 9 A mixture can be separated into its constituents by physical processes. A homogeneous solution tends to be identical, no matter how you sample. A mixture is a substance. When you combine two or more materials, you form a mixture. anne marie helmenstine, ph.d. We are able to see oil and water clearly separately in the. Mixture of oil and. Mixture Examples Class 9.
From www.teachoo.com
Differentiate b/w Homogeneous and Heterogeneous mixtures Teachoo Mixture Examples Class 9 A homogeneous solution tends to be identical, no matter how you sample. anne marie helmenstine, ph.d. by combining two or more substances, a mixture is produced. Updated on may 01, 2024. Mixture of oil and water is a heterogeneous mixture. the three phases or states of matter are gas, liquid, and solid. When you combine two or. Mixture Examples Class 9.
From mungfali.com
10 Examples Of Heterogeneous Mixture Mixture Examples Class 9 a mixture is a material that is made up of two more chemical compounds or substances that do not combine together. the three phases or states of matter are gas, liquid, and solid. The various substances present in a mixture are known as “constituents of the mixture” or “components of a mixture”. A homogeneous solution tends to be. Mixture Examples Class 9.
From www.youtube.com
What is an example of a mixture in everyday life Chemistry Class 9 Mixture Examples Class 9 by combining two or more substances, a mixture is produced. the three phases or states of matter are gas, liquid, and solid. When you combine two or more materials, you form a mixture. In chemistry, a mixture is a. Updated on may 01, 2024. We are able to see oil and water clearly separately in the. The various. Mixture Examples Class 9.
From coolschoolcomics.com
Mixtures and Solutions Lesson Plan Cool School Comics Mixture Examples Class 9 We are able to see oil and water clearly separately in the. A mixture can be separated into its constituents by physical processes. the three phases or states of matter are gas, liquid, and solid. by combining two or more substances, a mixture is produced. The various substances present in a mixture are known as “constituents of the. Mixture Examples Class 9.
From www.yourdictionary.com
Examples of Mixtures YourDictionary Mixture Examples Class 9 anne marie helmenstine, ph.d. A mixture can be separated into its constituents by physical processes. A mixture is a substance. When you combine two or more materials, you form a mixture. Updated on may 01, 2024. by combining two or more substances, a mixture is produced. a mixture is a material that is made up of two. Mixture Examples Class 9.
From chemistryclinic.co.uk
1.8 Elements, compounds, mixtures Chemistry Mixture Examples Class 9 the three phases or states of matter are gas, liquid, and solid. anne marie helmenstine, ph.d. by combining two or more substances, a mixture is produced. A homogeneous solution tends to be identical, no matter how you sample. In chemistry, a mixture is a. We are able to see oil and water clearly separately in the. A. Mixture Examples Class 9.
From sciencewithpizzi.weebly.com
1.4) Let's Focus on Compounds and Mixtures Science with Mrs Pizzimenti Mixture Examples Class 9 The various substances present in a mixture are known as “constituents of the mixture” or “components of a mixture”. In chemistry, a mixture is a. A homogeneous solution tends to be identical, no matter how you sample. by combining two or more substances, a mixture is produced. A mixture is a substance. We are able to see oil and. Mixture Examples Class 9.
From brainly.in
Give three differences between Homogeneous mixture and Heterogeneous Mixture Examples Class 9 a mixture is a material that is made up of two more chemical compounds or substances that do not combine together. the three phases or states of matter are gas, liquid, and solid. Updated on may 01, 2024. anne marie helmenstine, ph.d. Mixture of oil and water is a heterogeneous mixture. A homogeneous solution tends to be. Mixture Examples Class 9.
From www.teachoo.com
What is a Mixture? Types of Mixtures Chemistry Teachoo Mixture Examples Class 9 A homogeneous solution tends to be identical, no matter how you sample. Updated on may 01, 2024. We are able to see oil and water clearly separately in the. The various substances present in a mixture are known as “constituents of the mixture” or “components of a mixture”. Mixture of oil and water is a heterogeneous mixture. anne marie. Mixture Examples Class 9.
From exobkcoth.blob.core.windows.net
Definition Of Suspension In Chemistry Class 9 at Jeff Obryan blog Mixture Examples Class 9 by combining two or more substances, a mixture is produced. A mixture is a substance. a mixture is a material that is made up of two more chemical compounds or substances that do not combine together. anne marie helmenstine, ph.d. the three phases or states of matter are gas, liquid, and solid. In chemistry, a mixture. Mixture Examples Class 9.
From www.youtube.com
Difference between Mixture and Compound in Chemistry YouTube Mixture Examples Class 9 A mixture is a substance. When you combine two or more materials, you form a mixture. In chemistry, a mixture is a. the three phases or states of matter are gas, liquid, and solid. The various substances present in a mixture are known as “constituents of the mixture” or “components of a mixture”. We are able to see oil. Mixture Examples Class 9.
From animalia-life.club
Mixtures Examples Mixture Examples Class 9 Updated on may 01, 2024. In chemistry, a mixture is a. We are able to see oil and water clearly separately in the. A homogeneous solution tends to be identical, no matter how you sample. the three phases or states of matter are gas, liquid, and solid. a mixture is a material that is made up of two. Mixture Examples Class 9.
From in.pinterest.com
difference between compound and mixture Compounds and mixtures Mixture Examples Class 9 the three phases or states of matter are gas, liquid, and solid. When you combine two or more materials, you form a mixture. Mixture of oil and water is a heterogeneous mixture. We are able to see oil and water clearly separately in the. a mixture is a material that is made up of two more chemical compounds. Mixture Examples Class 9.
From kunduz.com
Heterogeneous Mixtures Definition, Properties, Types, Examples Mixture Examples Class 9 anne marie helmenstine, ph.d. by combining two or more substances, a mixture is produced. A homogeneous solution tends to be identical, no matter how you sample. the three phases or states of matter are gas, liquid, and solid. Updated on may 01, 2024. The various substances present in a mixture are known as “constituents of the mixture”. Mixture Examples Class 9.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Mixture Examples Class 9 A mixture can be separated into its constituents by physical processes. In chemistry, a mixture is a. Mixture of oil and water is a heterogeneous mixture. A mixture is a substance. A homogeneous solution tends to be identical, no matter how you sample. a mixture is a material that is made up of two more chemical compounds or substances. Mixture Examples Class 9.
From study.com
Homogeneous Mixture Definition Lesson for Kids Lesson Mixture Examples Class 9 In chemistry, a mixture is a. by combining two or more substances, a mixture is produced. the three phases or states of matter are gas, liquid, and solid. A mixture can be separated into its constituents by physical processes. Mixture of oil and water is a heterogeneous mixture. We are able to see oil and water clearly separately. Mixture Examples Class 9.
From www.yaclass.in
Types of mixture Homogeneous and Heterogeneous — lesson. Science State Mixture Examples Class 9 the three phases or states of matter are gas, liquid, and solid. anne marie helmenstine, ph.d. Updated on may 01, 2024. When you combine two or more materials, you form a mixture. We are able to see oil and water clearly separately in the. A mixture can be separated into its constituents by physical processes. In chemistry, a. Mixture Examples Class 9.